FOOD LABEL CHANGES: WHAT YOU
DON’T KNOW OR HAVEN’T THOUGHT ABOUT

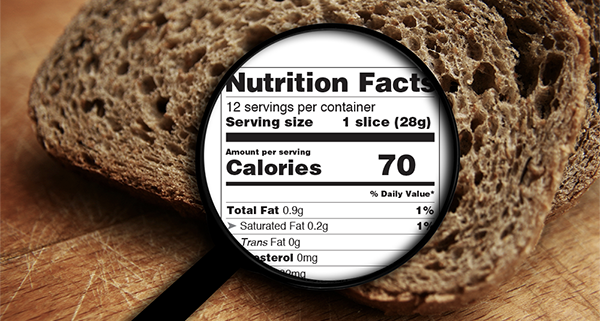
All of the changes implemented by the FDA will require changes to the nutritional label itself.
When the dual-column format must be used for Per Serving and Per Package food items, the entire package may need to be redesigned. These changes could take time to implement as a new design needs to be created and new plates made. If you haven’t started this process already, submit a contact form below and we’ll get you on the path to compliance today.
Effective Dates:
- January 1, 2020: Companies OVER $10 million in annual food sales
- January 1, 2021: Companies UNDER $10 million in annual food sales
- July 1, 2021 – Manufacturers of single-ingredient sugar products such as honey, syrup and certain cranberry-based foods
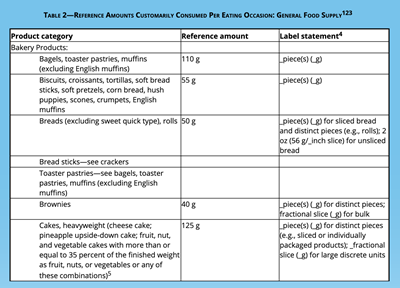 Increased Single Serving Size Amount:
Increased Single Serving Size Amount:
A Single Serving Size will be redefined as a serving that can reasonably be consumed by a single person in one sitting.
For example:
- A serving size of ice cream will increase from a half cup to 2/3 of a cup.
- Beverage cans and bottles will be counted as one serving because people tend to drink an entire beverage at one time, regardless of size.
For more specifics, visit the FDA’s RACC (Reference Amounts Customarily Consumed: CFR 21 §101.12) which is updated regularly.
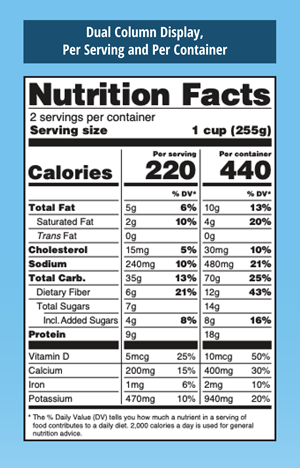
Dual-Column Data: Per Serving AND Per Package
Products that are larger than a single serving but could be consumed in a single sitting or multiple sittings must provide a dual column label to indicate the number of calories and nutrients on both a Per Serving AND Per Package or Per Unit basis.
IMPORTANT NOTE: The addition of the dual column format may require significant changes to the existing packaging design.
Other Important Changes:
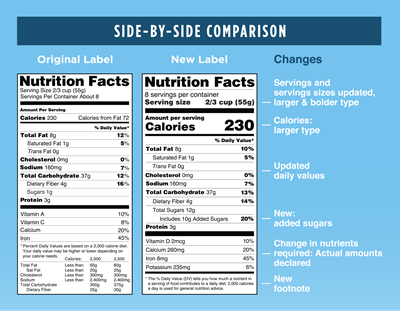
- Serving Size: Must be in large, bold, easy-to-read print
- Calories Per Serving: Will reflect new serving sizes and be in large, bold, easy-to-read print
- Calories From Fat: This section is being removed. However, Total Fat, Saturated Fat and Trans Fat will remain on the label.
- Added Sugars: Will be listed by weight and percent of daily value
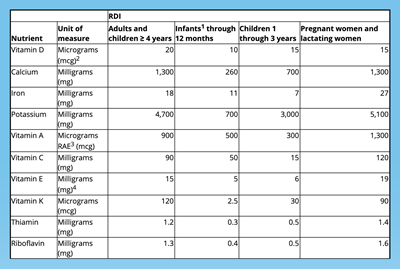
- Required Nutrient Changes: Must list the actual amount, in addition to percent Daily Value, of vitamin D, calcium, iron and potassium.
- Newly Required – Vitamin D and Potassium
- No Longer Required – Vitamins A and C
- Daily Values Updates: Daily values for nutrients like sodium, dietary fiber and vitamin D are being updated. The most up-to-date FDA charts detail nutritional values by age as well as segmenting out pregnant and lactating women.
-
The Footnote:
The small-print footnote at the bottom of the label will better explain the percentage of daily value and how it applies within the context of the total daily diet for the average person.The new footnote will read: The % Daily Value tells you how much a nutrient in a serving of food contributes to a daily diet. 2,000 calories a day is used for general nutrition advice.
Exemptions
Some manufacturers may be exempt from the labeling requirements, including low-volume producers with fewer than 100 full-time equivalent employees and moving less than 100,000 units per year.
Reference Information:
FDA Regulation on Food Labeling CFR Title 21.101
Reference Amounts Customarily Consumed (RACC)
FDA’s Quick Reference Guide of Changes
NEED TO CONTACT US?
Click HereNEED NEW ARTWORK FOR YOUR
LABEL OR SURROUNDING GRAPHICS?
The FDA regulated changes to your nutrition labels may not be as straight forward as they first appear. Uncovering the breadth of changes required for your packaging is no easy task.
Great American Packaging’s team of experts will guide you through the redesign process. We’ll make sure you have taken into account all the ways your packaging artwork and design will be impacted.
According to FDA guidelines, the new labels will include additional information about serving sizes, nutrients and calories, among other pertinent information. Font sizes and bolded text will also change. In some cases, a wider panel will be required to allow for additional columns of nutritional data.
We’ve identified a few ways to take advantage of the changes:
-
Consider refreshing your overall packaging design
Now is the perfect time to consider a packaging redesign. The FDA label changes will require new artwork and plates for your printed packaging. Save time and money by incorporating a new design at the same time.The company’s state-of-the-art technology, coupled with a team of experts, provides customers with a hassle-free experience when redesigning printed poly bags and films.
-
Analyze background choices
The most common background options – positive or reverse – make a difference in overall design clarity. Positive print (dark font on a light background) can be printed more clearly in smaller fonts, while reverse print (light font on a dark background) works better with larger typeface sizes and styles. For more information, download our guide to designing artwork. -
Consider your font
FDA guidelines state that any legible font can be used for the labels. Arial, Helvetica and Franklin Gothic are most common. Other easy-to-read fonts, including Verdana and Open Sans, are also readable.Keep in mind, the font size must be no less than one-sixteenth of an inch in height. (§101.2.c Information panel of package from food)
-
Use only high-quality printing technology
Misregistration due to poor trapping can result from the slightest movement of poly film on the press. Great American Packaging’s new EVO XD 8-color press prints vivid colors in a single pass, creating more sophisticated imaging and branding potential and eliminating the risk of poor trapping.
WE ARE GFSI CERTIFIED!

Great American Packaging is proud to be an IFS PacSecure certified facility – with a 97% rating. Our GFSI-recognized certification means clients can be sure that their packaging materials meet regulatory and safety standards. IFS facilitates B2B trade and helps improve product integrity along the entire supply chain.
Contact
"*" indicates required fields


